Students share experiences of COVID-19 vaccine
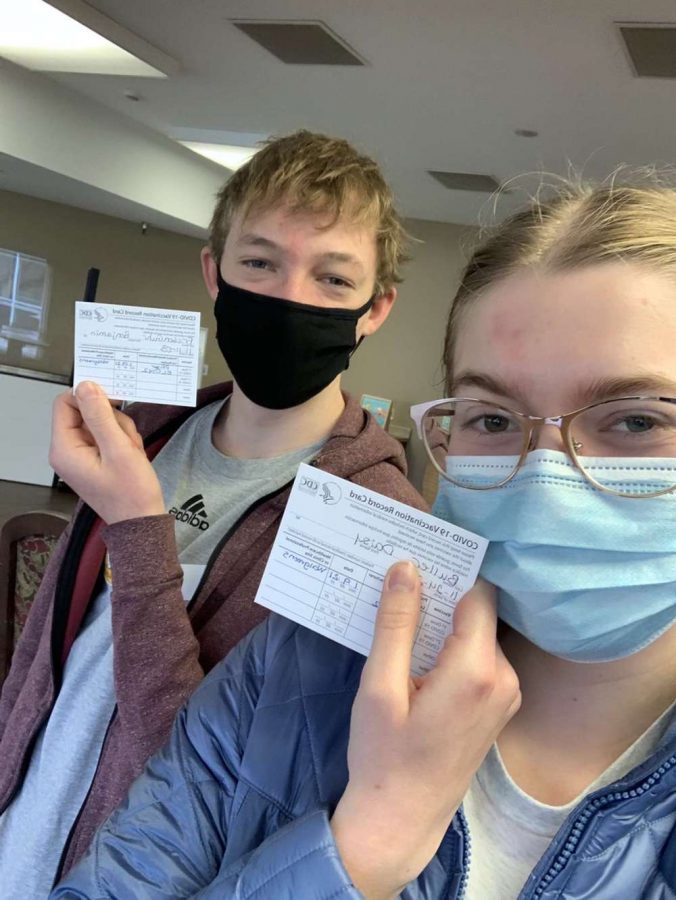
Juniors Ben Friesen Guhr (left) and Daisy Buller (right) pose with their COVID-19 Vaccination Record Card after receiving their first dosage of the vaccine.
January 28, 2021
Throughout November and December 2020 talk of a vaccine for COVID-19 being approved and released was big news to Americans, who have been dealing with the pandemic since February. Although some Americans have been able to receive the vaccine, most have to wait until there is enough vaccine to be given to the general public.
Vaccines are being given in phases, and high school students are not at the top of the list. First being given the vaccine are healthcare workers and long term care facility residents, then frontline essential workers and people of ages 75 years and older. Then people aged 65-74 years and people aged 16-64 years with underlying medical conditions or other essential workers. Finally, when enough of the vaccine has been created and sent to facilities, the general public will get to decide whether or not they will receive the vaccine.
“When I hope [the vaccine] will be administered, and when it really will be are two very different things,” school nurse Karen Lehman said. “I think it will be awhile before it is available to everyone, maybe by summer or next fall.”
Currently there are two vaccines that are approved and being given out to healthcare workers and long term care facility residents. The vaccines are made by Pfizer and BioNTech. The separate vaccines are not to be mixed per the Center for Disease Control and Prevention (CDC). According to the CDC, both vaccines are mRNA vaccines, which means that the vaccine uses a natural chemical called messenger RNA to produce an immune response. Both vaccines also have two shots, given 21 days apart in the muscle of the upper arm. Lehman has not received her vaccine yet, but plans to get it when available to her. Although, some of Lehman’s friends/family have received the vaccine.
“After receiving the vaccine my peers had no complications and are feeling fine,” Lehman said.
The CDC recommends most people be vaccinated, however there are few exceptions. If you receive a severe or non severe allergic reaction after getting the first dose of the vaccine, you should not receive your second dose. If you have any severe or non severe allergies to any ingredient in the vaccine, you should not receive the vaccine. People who are allergic to PEG or polysorbate should not get the vaccine. An immediate allergic reaction means a reaction within four hours of receiving the vaccine, including symptoms such as hives, swelling or wheezing, all according to the CDC. Junior Benjamin Friesen Guhr received the COVID-19 vaccine on Jan. 9.
“I did not have any allergic reactions to the COVID-19 vaccine after receiving it,” Friesen Guhr said.
Some Americans believe that since the COVID-19 vaccine was developed and tested rapidly, it is not safe or healthy. According to Mayo Clinic, many pharmaceutical companies invested significant resources into quickly developing a vaccine for the COVID-19 pandemic. Since this is an emergency situation, an emergency response was taken, but this does not mean that safety protocols were dismissed. The vaccine by both Pfizer and BioNTech has been studied in approximately 43,000 people, and the safety of the COVID-19 vaccine is being closely monitored by the CDC and the Food and Drug Administration (FDA).
“I trust the vaccine because the health professionals would be the people that know more about the subject than anyone and they have been working nonstop for months,” Friesen Guhr said.
It is safe to say that the COVID-19 vaccine can be trusted among the general public when we reach that point due to the high amounts of research and preparation by medical professionals. When the vaccine is ready to be given out to the public, it is suggested that each individual do their own research before receiving the vaccine to make sure it is the right decision for them.
“I decided to get the vaccine because it is important for ending the pandemic and keeping people safe,” Friesen Guhr said.



















Maria Longenecker • Oct 2, 2024 at 8:34 pm
Love to see it!!